 BBC
BBCA “Trojan horse” therapy that sneaks toxic drugs inside cancer cells is being made available on the NHS in England in a world first.
It can halt the blood cancer myeloma for nearly three times longer than current therapies.
The drug is an advanced form of chemotherapy that hits cancer with a bigger dose, while reducing side-effects.
Paul Silvester, one of the first people to get it, says the therapy has been “life-changing” and he’s now planning history-themed adventures.
Myeloma – also known as multiple myeloma – affects part of the immune system called plasma cells. These are made in the spongey bone marrow in the centre of our bones.
Paul, who is 60 and from Sheffield, was diagnosed nearly two years ago after the cancer led to broken bones in his back.
He had a bone marrow transplant last year, but relapsed around Christmas. He has since been on the new therapy – called belantamab mafodotin – as part of an early access scheme.
Within weeks he was in remission.
 Paul Silvester
Paul SilvesterOther treatments could have left him isolating in his bedroom for months, so Paul says the therapy “is absolutely life-changing” and was “creating that opportunity to enjoy” life.
Visiting Hadrian’s Wall is next on the agenda for history buff Paul; and he’s looking forward to one of his daughters graduating later this year.
“Most people say ‘you look really really well’… I have a good normal life,” he told the BBC.
How does this therapy work?
Paul’s therapy – belantamab mafodotin – is a lethal chemotherapy drug that has been bound to an antibody, similar to the ones the body uses to fight infection.
However, these antibodies have been designed to spot markings on the outside of plasma cells.
So they travel to cancerous cells, stick to the surface and are then absorbed. Once inside they release their toxic payload, to kill the cancer.
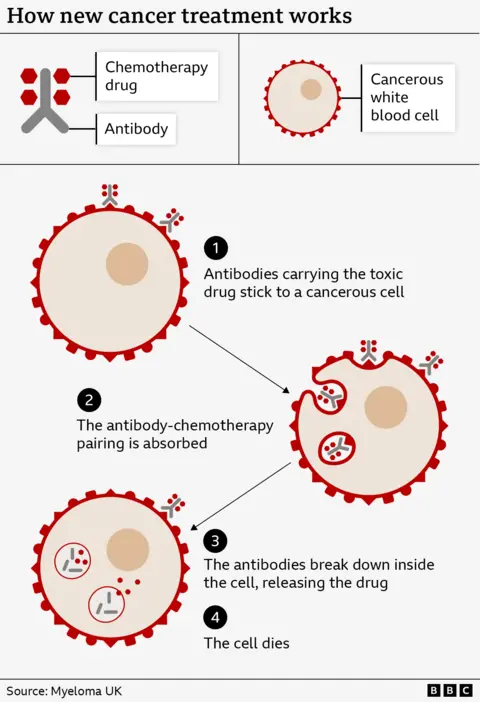
The therapy is named Trojan horse therapy after the siege of the city of Troy in Greek mythology, when a giant wooden horse was used to smuggle soldiers into the city.
Myeloma cannot be cured, but clinical trials last year showed the Trojan horse therapy halted the cancer for three years, compared to 13 months with current therapies.
Prof Peter Johnson, the national clinical director for cancer at NHS England, said the difference was “life-changing”.
He told me: “This is a really important development for people with myeloma, because although we may not be able to cure the illness, giving them time free of the disease and free of the symptoms is really important.
“We’ve seen in the last few years that using antibodies to deliver chemotherapy drugs directly into cells can make a big difference for a variety of different types of cancer.”
 Paul Silvester
Paul SilvesterAround 33,000 people are living with myeloma in the UK. The new drug will be used when the first-choice therapy fails, so around 1,500 patients a year could benefit.
The decision comes after a review by the National Institute of Health and Care Excellence (NICE) concluded the drug was cost-effective for NHS use. NICE recommendations are normally adopted in England, Wales and Northern Ireland while Scotland has its own process.
The therapy is kinder than other cancer treatments, but is not free from side-effects.
After a cancer cell has been destroyed, the remaining chemotherapy drug will leak into the body. This can cause dry eyes and blurred vision.

‘These are very smart drugs’
The technical name for these drugs is an antibody-drug-conjugate.
This therapy was developed by GSK in the UK with early research taking place in Stevenage and the first clinical trials in London.
Prof Martin Kaiser, team leader in myeloma molecular therapy at the Institute of Cancer Research, said these “are very smart drugs” and the difference in side effects compared to other drugs “is really remarkable”.
While myeloma is still considered an incurable cancer, Prof Kaiser says drugs like this are “an important step towards a functional cure” and he thinks long-term remission will go “above 50% in the next five years”.
Antibody drug conjugates are being developed for a range of cancers. The limitation is being able to design an antibody that can target the cancer alone. There is one that can target some types of breast cancer. Research is already taking place on stomach and bowel cancer.
Shelagh McKinlay, from the charity Myeloma UK, said the approval would “transform the lives of thousands” and it was “fantastic to see the UK at the forefront of myeloma treatment”.
Health Minister Karin Smyth, said: “This ground-breaking therapy puts the NHS at the forefront of cancer innovation.”
www.bbc.com
#Worldfirst #Trojan #horse #therapy #NHS






